The brief addresses the effectiveness of existing and upcoming vaccines against emerging COVID-19 viral variants and provides considerations for Governors.
Introduction
Vaccines are critical tools that can reduce COVID-19 disease and help end the pandemic. The Biden Administration has set a goal to have 300 million individuals vaccinated across the United States by July 2021 and Governors are at the forefront of this vaccine distribution effort. Currently, approximately 2.16 million individuals are vaccinated every day and as supply increases, states and other providers will need to collectively accelerate vaccinations to an estimated 3 million per day to reach the 300 million goal on time. Adding urgency to this challenge, new and more transmissible COVID-19 viral variants are spreading across the country, increasing pressure on states to quickly vaccinate as many individuals as possible, while ensuring equity and maintaining other mitigation measures to limit viral transmission.
Although current vaccine supply is limited, production has been steadily increasing and the federal government has indicated there will be enough vaccine to vaccinate all adults in the U.S. by the end of May. Johnson & Johnson’s COVID-19 vaccine recently received emergency authorization and promising candidates from Novavax and AstraZeneca are nearing U.S. Food and Drug Administration (FDA) review. This issue brief provides an overview of current evidence about the safety and efficacy of Johnson & Johnson’s recently authorized vaccine and additional vaccine candidates anticipated to receive near-term emergency authorization from the FDA. The brief also addresses the effectiveness of existing and upcoming vaccines against emerging COVID-19 viral variants, including what is known based on interim clinical trial results and preliminary studies and how Governors can address variants in the short- and long-term.
Johnson & Johnson’s Vaccine Authorization and Prospective COVID-19 Vaccines
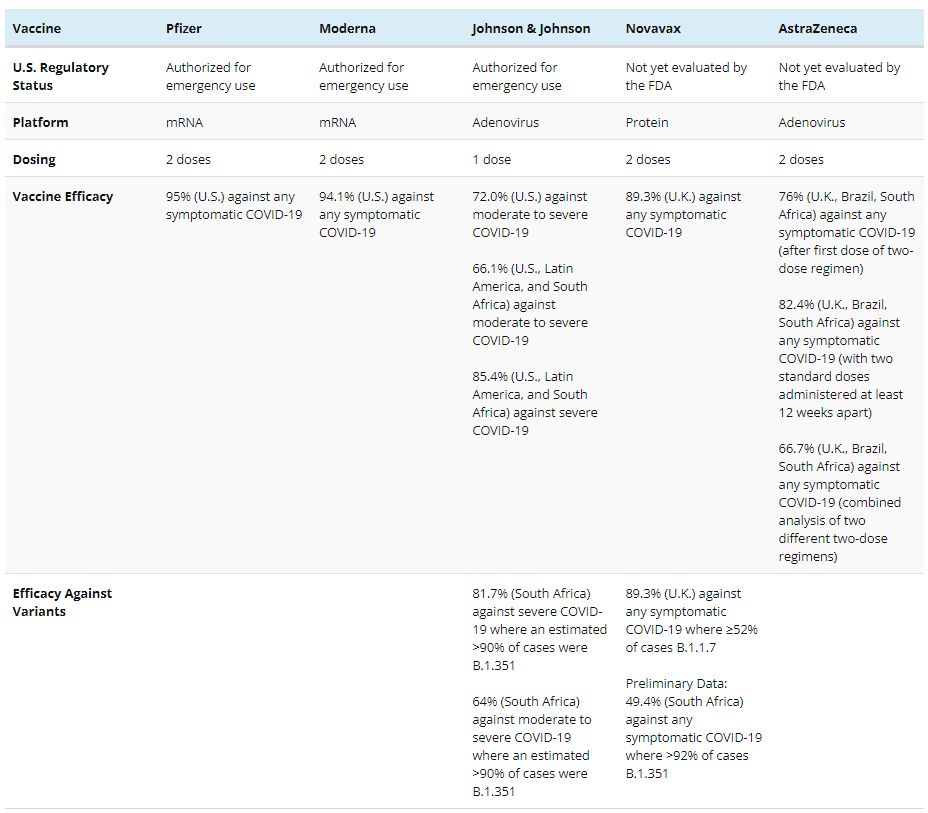
On February 27, the FDA granted an emergency use authorization (EUA) for Johnson & Johnson’s COVID-19 vaccine for single-dose vaccination, following the independent Vaccines and Related Biological Products Advisory Committee’s (VRBPAC) unanimous vote recommending authorization. Data from Johnson & Johnson’s large ongoing clinical trial for its one-dose vaccine regimen raised no significant safety concerns and indicated 72.0% efficacy against moderate to severe COVID-19 and 85.9% efficacy against severe COVID-19 among U.S. participants. Reported efficacy against moderate to severe COVID-19 fell to 68.1% in Brazil and 64.0% in South Africa, with the prevalence of concerning viral variants in those locations likely a contributing factor. The Centers for Disease Control and Prevention (CDC)’s Advisory Committee on Immunization Practices (ACIP) has advised that the Johnson & Johnson vaccine be used for individuals 18 years of age and older. Accordingly, state policymakers have started deploying this as a third authorized COVID-19 vaccine. Johnson & Johnson will deliver 3.9 million vaccine doses immediately and expects to deliver more than 20 million by the end of March 2021.
Additional COVID-19 vaccine candidates from Novavax and AstraZeneca are under investigation in ongoing U.S. clinical trials and may soon be authorized for emergency use. Initial reports of vaccine efficacy from clinical trials outside the U.S. indicate both vaccines are promising. The two-dose Novavax vaccine regimen showed 89.3% efficacy against symptomatic COVID-19 disease, and a two-dose AstraZeneca vaccine regimen showed 82.4% efficacy against symptomatic COVID-19 disease when doses were administered at least 12 weeks apart. How the vaccines from Novavax and AstraZeneca protect against emerging viral variants remains under investigation and is discussed in detail below.
Before the FDA grants emergency authorization to either the Novavax or AstraZeneca vaccine, both companies need to provide data from larger U.S. clinical trials. These additional data can better characterize the overall safety and efficacy of each vaccine in the U.S. context. While initial data from Novavax’s smaller U.K. and South Africa studies is promising, the ongoing U.S. clinical trial (PREVENT-19) is substantially larger and is expected to report initial data in early April 2021.
Emergency authorization of the AstraZeneca vaccine likewise depends on data from an ongoing U.S. clinical trial. Interim efficacy estimates from AstraZeneca’s large clinical trial in the U.K., Brazil, and South Africa indicated 66.7% efficacy against any symptomatic COVID-19 among participants who received either two standard doses or a low-dose followed by a standard dose. However, efficacy against the same outcome increased to 82.4% among a smaller set of participants who received two standard doses at least 12 weeks apart. A small clinical trial in South Africa also raised questions about the vaccine’s efficacy against mild to moderate COVID-19 among those exposed to a particular viral variant and prompted the South African government to delay distribution. However, this smaller clinical trial did not evaluate efficacy against severe disease and does not reflect the U.S. context, having evaluated AstraZeneca’s vaccine among only 2,000 participants with an average age of 31. Ultimately, although efficacy estimates have varied among different vaccination regimens and participant populations, additional evidence about the vaccine’s optimal dosing regimen, efficacy against severe disease, and efficacy among people over 65 is expected from the ongoing U.S. clinical trial. The FDA anticipates data from AstraZeneca’s U.S. clinical trial might clarify these issues and interim analyses may arrive in March or April 2021.
In addition to increasing existing vaccine supply, emergency authorization of additional vaccines can provide options for states and providers challenged by the complex handling and storage requirements of previously authorized vaccines. Johnson & Johnson, Novavax, and AstraZeneca have developed vaccines that can be stored with standard refrigeration, and the Pfizer vaccine can now alternatively be distributed and stored at standard freezer temperature. Notably, the Johnson & Johnson vaccine requires only a single dose, alleviating logistical challenges posed by two-dose regimens. And simplified storage and handling requirements will ease vaccine distribution among a wider variety of community settings (like primary care offices) and within underserved communities.
Policymakers need to weigh both real and perceived differences between vaccines as they prioritize populations for vaccination and design communications to build trust among communities. Questions are already being raised about differences between vaccines and might contribute to vaccine hesitancy if they are not adequately addressed. Given the evidence to date, messaging can reinforce that although the Johnson & Johnson vaccine’s estimated overall efficacy against moderate to severe COVID-19 may not match the efficacy reported by Pfizer and Moderna, the different clinical trials are not directly comparable and all three vaccines are highly effective at preventing severe disease and death from COVID-19. And based on early data, upcoming vaccines from Novavax and AstraZeneca may be similar. Cases of severe COVID-19 among vaccinated participants have been very rare across all clinical trials of vaccines from Johnson & Johnson, Novavax, and AstraZeneca, suggesting each vaccines’ potential to prevent the worst of COVID-19.
Emerging COVID-19 Variants in the United States
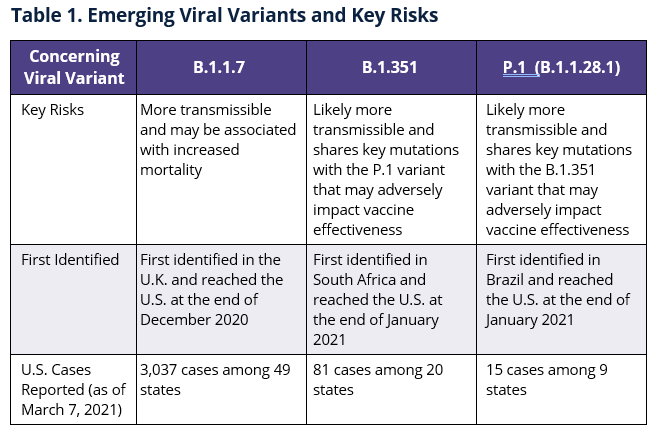
At least three emerging variants of the virus that causes COVID-19 (B.1.1.7, B.1.351, and P.1) are spreading in the U.S., raising concerns about potential increases in outbreaks and reduced vaccine effectiveness. Early studies indicate each variant may be more transmissible and there are also concerns about whether some variants might increase severe COVID-19 and overall morbidity and mortality. Accordingly, genomic surveillance capabilities are being expanded to monitor these and other emerging variants and the CDC anticipates that the B.1.1.7 variant may become dominant in the U.S. by the end of March 2021.
Effectiveness of Current and Prospective COVID-19 Vaccines Against Viral Variants
Based on available data, current COVID-19 vaccines remain effective against concerning viral variants. However, they may be less effective in preventing asymptomatic and mild COVID-19 among those infected with concerning viral variants. Clinical trials for Pfizer and Moderna’s vaccines occurred in the U.S. before the emergence of concerning variants, and consequently did not report data reflecting vaccine efficacy against emerging viral variants. However, preliminary laboratory-based studies indicate both the Pfizer and Moderna vaccines will likely prevent severe COVID-19, hospitalization, and death among those infected with the B.1.1.7. and B.1.351 variants. Results from these same laboratory studies also suggest that vaccine-elicited immunity to the B.1.351 variant might wane more quickly than immunity to other variants.
Additionally, while the P.1 variant was only recently identified, key mutations shared among the P.1 and B.1.351 variants suggest that vaccine effectiveness against these variants may be similar. In response, Moderna is testing the effect of adding a third dose to its vaccine regimen (a booster shot) and has developed a version of its vaccine that targets the B.1.351 variant specifically. Pfizer is also testing the effect of adding a third dose to its vaccine regimen and is preparing to study a variant-specific vaccine. Pfizer will likely announce more information on their variant-specific vaccine in the coming weeks.
Recent clinical trial results from Johnson & Johnson and Novavax are the first to describe vaccine efficacy among clinical trial participants who became infected with concerning viral variants (specifically, B.1.351 and B.1.1.7). Overall efficacy estimates appeared less than that of the vaccines from Pfizer and Moderna, particularly in locations where the B.1.1.7 and B.1.351 viral variants were dominant (see Table 2). As a result, reduced efficacy compared to the Pfizer and Moderna vaccines may be due to the effect of viral variants.
However, because clinical trials for the vaccines from Pfizer and Moderna occurred in the U.S. before the emergence of concerning viral variants, comparing the efficacy between these vaccines and vaccines from Johnson & Johnson and Novavax is difficult. Until additional ongoing studies report results about the Pfizer and Moderna vaccines among populations exposed to the B.1.1.7 and B.1.351 variants, information about their real-world effectiveness against such variants remains limited.
Early results indicate that Johnson & Johnson’s recently authorized vaccine can prevent severe COVID-19, hospitalization, and death to a substantial degree, even among those infected with concerning viral variants, despite reduced overall efficacy estimates. The Johnson & Johnson vaccine prevented 85% of severe disease 28 days post-vaccination across all locations and no cases of severe COVID-19 were reported 49 days post-vaccination. And early results for Novavax’s vaccine candidate similarly indicate substantial protection against severe COVID-19, hospitalization, and death. Furthermore, a recent real-world vaccine effectiveness study among 5.4 million people in Scotland indicates the Pfizer and AstraZeneca vaccines had an 85% and 94% effect preventing hospitalization 28 to 34 days after vaccinations, respectively. The same vaccine effect was 81% among those over 80 years of age who received either vaccine.
These results reinforce the potential for widespread vaccination to alleviate the worst of COVID-19 regardless of the vaccine used. However, if concerning viral variants become widespread, both currently authorized and upcoming vaccines may afford less protection against mild to moderate COVID-19, in which case adding booster doses to existing regimens or updating vaccines may be warranted.
Whether vaccines effectively prevent asymptomatic infections and viral transmission remains largely unknown, but initial studies indicate vaccines likely mitigate some viral transmission. Johnson & Johnson’s EUA request included interim clinical trial data based on limited participant follow-up suggesting their vaccine may be between 46.8 to 88.4% efficacious against asymptomatic infections 29 days following vaccination. And Pfizer’s vaccine might likewise reduce asymptomatic infections and viral transmission according to two recent studies conducted in Israel. If vaccines are ultimately able to reduce viral transmission, the benefit of quickly vaccinating the U.S. population can extend beyond preventing severe COVID-19 to mitigating future outbreaks as well as threats posed by concerning viral variants.
Table 2: COVID-19 Vaccine Efficacy as Reported Across Clinical Trials
Considerations for Governors
Governors, policymakers, and public health officials can consider the following as they update vaccination strategies and public health communications to reflect new information about upcoming vaccines and emerging viral variants:
Recently authorized vaccines provide substantial protection against severe COVID-19, hospitalizations, and death and upcoming vaccines are showing similarly promising results. Although overall estimates of efficacy against any symptomatic COVID-19 appear reduced among upcoming vaccines as compared to efficacy estimates from Pfizer and Moderna, Johnson & Johnson’s recently authorized vaccine and vaccines from Novavax and AstraZeneca are all expected to prevent severe COVID-19. The Johnson & Johnson vaccine showed 85% efficacy against severe COVID-19 in its U.S. clinical trial. While complete clinical trial data from Novavax and AstraZeneca has not yet been released, there are no reported cases of severe COVID-19 among fully-vaccinated participants according to the latest interim clinical trial data.
Public health communications must acknowledge that COVID-19 vaccines differ in some ways while reinforcing key messages about their effectiveness and the importance of vaccination. Because the media has widely reported various figures characterizing vaccine efficacy among upcoming vaccines (see Table 2), individuals are understandably concerned they might receive a less effective COVID-19 vaccine. Public health communications can acknowledge that vaccine effectiveness is measured against different outcomes, but the primary goal of the COVID-19 vaccination effort is to prevent severe COVID-19, hospitalization and death and that all authorized vaccines are highly efficacious against these outcomes. These messages emphasize the value of vaccination, no matter the COVID-19 vaccine received. Furthermore, Governors and public health leaders can consider ways to increase transparency in vaccine allocations and can engage community and health care leaders to guide strategies to distribute new vaccines, particularly among at-risk and underserved communities.
Vaccine supply is increasing and states can expect to increase vaccinations and incorporate more easily distributed vaccines. The Biden administration anticipates delivering enough supply to vaccinate all adults in the U.S. by the end of May 2021. In the near-term, both Pfizer and Moderna are increasing vaccine production and delivery and the supply of Johnson & Johnson’s vaccine is expected to significantly increase in March and April. The ACIP recently recommended Johnson & Johnson’s single-dose, refrigerator-stable vaccine for individuals 18 years of age and older. Accordingly, states can begin planning distribution strategies that consider both the potential for wider deployment of refrigerated vaccines among community settings that are not equipped to handle ultra-cold storage, as well as vaccinating populations that can benefit from a single-dose regimen. Furthermore, the FDA announced that the Pfizer vaccine can now be transported and stored at conventional freezer temperatures for up to two weeks before thawing for dilution and administration. This update enables the distribution and storage of the Pfizer vaccine among additional community-based settings where standard freezers are common.
Public health interventions meant to reduce viral transmission remain critical, especially to combat emerging viral variants. Continued and widespread mask-wearing and physical distancing can reduce viral transmission, slowing both the spread and emergence of concerning viral variants. Mask-wearing and physical distancing remain important for three additional reasons: how vaccines may reduce viral transmission remains an open question, the duration of protection among vaccines is not yet known, and vaccine effectiveness against viral variants remains poorly understood. Accordingly, communications ought to encourage mask-wearing, even among vaccinated individuals until additional evidence is developed.
Vaccination strategies may eventually include booster doses or updated vaccines to address waning immunity or concerning viral variants. Preliminary studies and interim clinical trial data indicate concerning viral variants may reduce vaccine effectiveness and it’s not clear how long vaccines provide protection against COVID-19. For these reasons, manufacturers are already testing the potential benefit of adding booster doses to existing regimens and designing vaccines that address specific mutations among concerning viral variants. While studies characterizing vaccines’ durations of protection are ongoing, experts are already suggesting that public health authorities will likely recommend booster doses later this year. As states continue to advance vaccine distribution plans, partnerships, and data systems, Governors and public health leaders should consider the likelihood that individuals may need to be reengaged to receive future booster vaccinations.
Clinical trials are needed for children and adolescents. While COVID-19 does not seem to harm children as severely as adults, it is important to conduct pediatric clinical trials to determine optimal vaccine dosing and to evaluate vaccine safety among children and adolescents. Pediatric clinical trials are now underway. Pfizer completed enrollment for a clinical trial including 12 to 16-year old children and began a study include 5 to 12 year old children. Last month, Moderna started a clinical trial for 12 to 17 year old adolescents, but recruitment is ongoing to enroll an adequate clinical trial population.
Authors
This publication was developed by Nicholas Harrison, Hemi Tewarson and Katie Greene at the Duke-Margolis Center for Health Policy, in partnership with Kate Johnson at the National Governors Association Center for Best Practices.
Recommended Citation Format
Harrison, N., Tewarson, H., Greene, K., Johnson, K. (2021, March). COVID-19 Vaccines and Emerging Viral Variants: Considerations for Governors, Washington, DC: Duke-Margolis Center for Health Policy and the National Governors Association Center for Best Practices.